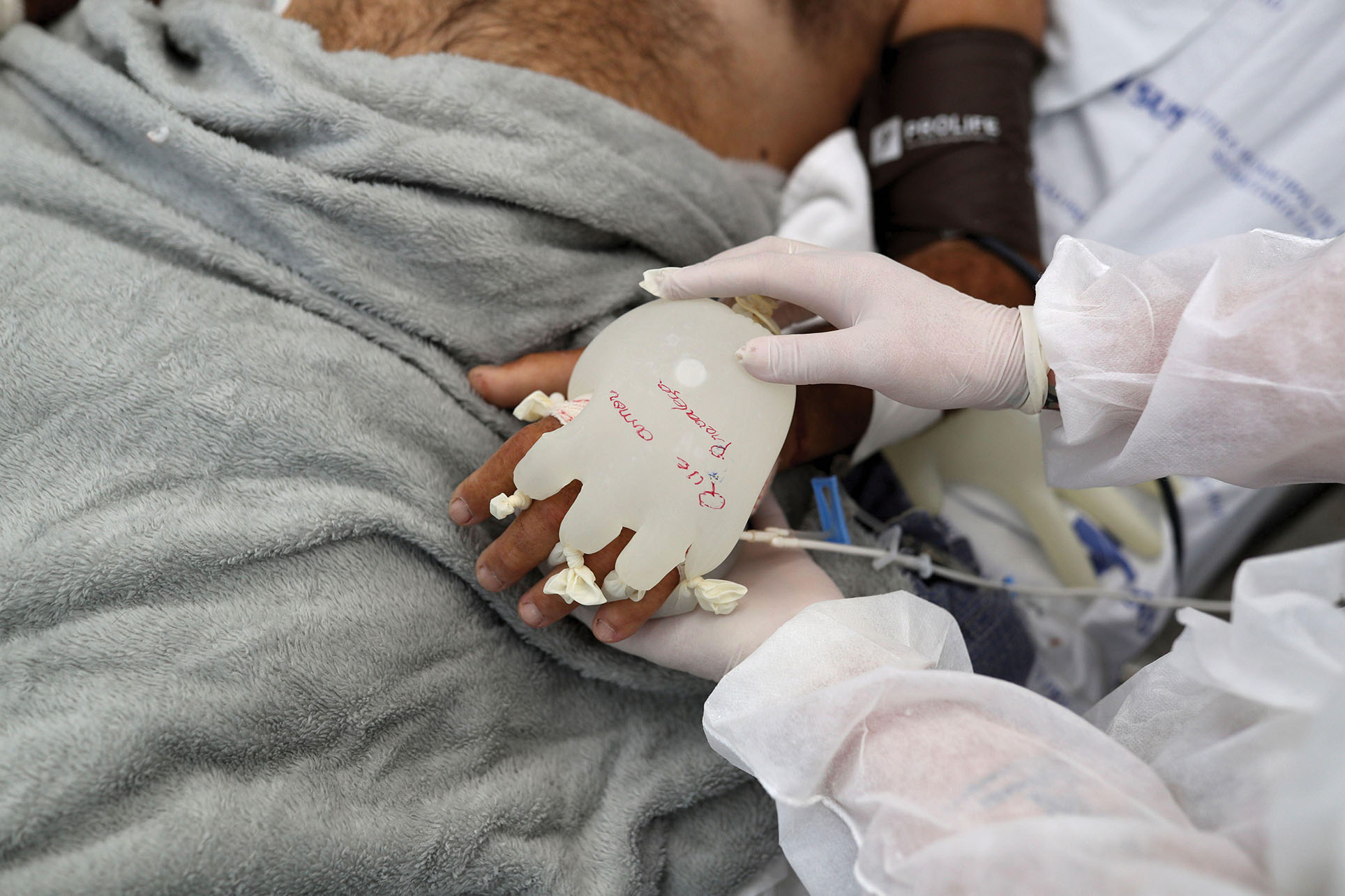
At first glance, the image is peculiar: a patient’s hand, at repose in a hospital bed, covered by an engorged latex glove with knotted fingertips and wrist. The mãozinha (little hand) is full of warm water to mimic the warmth and weight of human touch and give comfort to a critical Covid-19 patient in São Paulo, Brazil. In hospitals throughout Latin America, patients lie intubated, alone and cold. Yet health care workers persist, innovating, managing tragedy with ingenious adaptations, and applying brute human force.
In the year 2020, Latin America and the Caribbean bore 28 percent of the world’s confirmed Covid deaths while representing just 8.4 percent of the global population. While inconsistencies in national reporting practices and testing rates cast some doubt on this statistical map of death, whatever the approach used to analyze the global data, Covid has been devastating for the region.
The pandemic came to Latin America in a context of social, political, and economic instability. At the start of 2020, global economic demands shifted away from the commodity boom that shaped the turn of the 21st century. With the end of a global super cycle, the region grappled with a number of social and economic issues: stubborn structural poverty, surging political instability, a refugee crisis of historic dimensions, general social unease, weak institutions, fledgling political parties, and an information environment of rampant misinformation. The Covid crisis exacerbated many of these deep challenges.
At the same time, Covid shed light on an immediate problem for Latin America: throughout the region, governments struggled to source and supply medicine. Irrespective of the policy tools deployed to contain the pandemic from a public health perspective, all countries in Latin America faced a shared challenge in sourcing the pharmaceutical inputs required to launch systematic, effective public responses.
The root of the region’s supply fragility is its distinctly import-dependent structure, with scant basic pharmaceutical production, heavy generics concentration, and nascent biopharmaceutical capabilities. Pharmaceutical industries represent a far smaller portion of gross domestic product in Latin America and the Caribbean than in other member countries of the Organisation for Economic Co-operation and Development (OECD). In 2018, the region had a pharmaceutical trade deficit of nearly US$21 billion. This figure has exhibited steady growth over the past 30 years.
As an import-dependent region, Latin American governments reeled in the face of global shortages as the pandemic peaked again and again. Crucially, local capacity has not been able to scale up to meet domestic demands. In March 2021, for example, more than a year into the pandemic, Brazil faced shortages of anesthetics, sedatives, neuromuscular blockers, and pain medicines (O Globo, 2021). In Honduras, doctors have taken to Twitter to expose the desperate situation in public hospitals (Sosa, 2021). In Peru, doctors launched hunger strikes to call attention to the shortages. We have anecdotal evidence throughout the region of supply disruptions for key pharmaceutical agents crucial for artificial ventilation, though as of yet there has been no full regional review.
Still, we know that global health inequalities have been in dramatic evidence throughout the Covid crisis (Bollyky et al., 2020). High dependence on imports of medicines generally and Covid critical-care products specifically have constrained the ability of governments to guarantee essential personal protective equipment for healthcare workers, enable access to tests, provide respirators and oxygen when required, and rally the arsenal of pharmacological agents used to combat the effects of Covid-19 (Delgado, 2020). While there is no cure for the SARS-CoV-2 virus that causes Covid-19, three core pathophysiological processes triggered by the disease — severe hypoxemia, hyperinflammation, and hypercoagulability — can be combated with a protocol of pharmacological agents to reduce mortality rates (Ali et al., 2020; Benavides-Cordoba, 2020; Canedo-Marroquín et al., 2020). Though these medicines have been identified and their efficacy established, governments globally, but especially in Latin America, have struggled to coordinate a complex web of systems and procure the inputs necessary to offer treatment to their citizens (Socal et al., 2021).
Even in Latin America’s most technologically advanced countries, which boast extensive systems of pharmaceutical production, ensuring the supply of key pharmaceutical agents has proven challenging. Lack of medicine has affected not only those suffering from Covid, but those battling other diseases, as well. From oxygen and masks, to anesthesiology medications, to vaccines, millions of Latin Americans experienced the scarcity of medicine over the past year. Most alarmingly, as of this writing, the path to mass vaccination in many Latin American countries remains uneven and tenuous.
Scarcity is reshaping the debate among those of us working on issues related to access to medicine and innovation in Latin America. How has Covid highlighted weaknesses in the structure of medical innovation and delivery systems in the region? What are the barriers and opportunities currently being examined in academic and policy circles? How do we rethink and rebuild access to medicine in a context of crisis?

One interesting aspect of the supply challenge for Latin American governments is that in many streams of pharmacologic products, key medicines are no longer protected by patents. In principle, generic alternatives should be ubiquitous and available at low bulk prices. That Brazil — one of the developing world’s leading pharmaceutical producers, globally heralded for its local production of HIV/AIDS medicines and for its response to Zika — should be constrained by a scarcity of such basic inputs is illustrative of the challenge of pharmaceutical sourcing during a global pandemic.
Over the past three decades, a great deal of scholarship on access to medicine in developing countries has worked to unravel the role of intellectual property (Roffe et al., 2005; Sell, 2003). The “harmonization” of intellectual property systems through the intellectual property rights regime of the World Trade Organization (WTO) was originally touted by its supporters to be key to equalization. At the start of global trade negotiations in the late 1980s, the global pharmaceutical industry argued that lack of innovative output in the developing world was due to weak institutions, namely intellectual property rights. With Latin America’s assent to the terms set out in the WTO Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS), the region expected to see increased investment in innovative activities.
Scholarship and on-the-ground policy work in subsequent years cast doubt on that argument. More rigid and longer intellectual property rights do not increase innovative output in developing countries (Sweet & Eterovic, 2015). In fact, they likely reduced access to medicines in the Global South. In the late 1990s, several large and leading developing countries — most notably South Africa and Brazil — led a pushback. Both countries utilized flexibilities in the agreement’s Article 31 and issued compulsory licenses on components of widely used antiretrovirals for HIV/AIDS patents (Correa, 2015). Under TRIPS Article 31, governments can give authorization to use a patented invention without the consent of the patent-holder under specific conditions, such as a public health emergency like the coronavirus pandemic. The authorization given by compulsory licensing cannot be exclusive to any particular manufacturer and is intended to supply the domestic market only.
Brazil could employ this mechanism because it had the political capital and local production capacity to back up the policy. Still, it was constrained by not locally producing active pharmaceutical ingredients for antiretrovirals. These first “building blocks” were sourced from suppliers in India and subsequently formulated into finished medicines in Brazil’s public laboratories. According to the World Health Organization (WHO), Brazil’s leadership made this Latin American country a “trailblazer” (WHO, 2018).
By mid-May 2021, the politics of intellectual property in the battle to secure access to a coronavirus vaccine fully unfolded (WHO, 2021). Turning away from previous U.S. policy, Kathleen Tai, the Biden administration’s representative to the WTO, issued a statement that the United States would endorse use of patent flexibilities: “The Administration believes strongly in intellectual property protections, but in service of ending this pandemic, supports the waiver of those protections for Covid-19 vaccines. We will actively participate in text-based negotiations at the WTO needed to make that happen” (Tai, 2021). Within 48 hours of the reversed U.S. position, the Europeans — led by Angela Merkel — dismissed this position, arguing that it would result simply in a transfer of technology to Russia and China and not supercharge efforts to distribute the vaccine globally. Many looked skeptically at the United States, who had shared vaccines globally at a comparatively miniscule rate in contrast to European partners. Whether or not this moment signals an enduring policy shift for the United States or an exceptional and fleeting moment, a division between these two groups reflects the larger geopolitical power clash regarding global vaccine distribution.
In Latin America, contrasting visions emerged. Brazil, once the leader in the push for intellectual property flexibilities, spurned the idea of issuing compulsory licenses outright. Foreign Minister Carlos Alberto de Franco França told a Brazilian Senate committee in early May, “vaccines are almost impossible to copy, in the short or medium term, without the support of the laboratories that developed them — even with the aid of the patent.” According to França, “The biggest bottleneck today, for access to vaccines, is the material limits of production capacity” (Chazan et al., 2021).

While the politics of intellectual property are once again making headlines in Latin America, the crisis has put in stark relief how the rules governing innovation are one piece in a complex institutional and production landscape. To ensure access to medicine, governments in the region must address multiple inputs across the sector and a kaleidoscope of policy areas including: regulatory frameworks; public procurement and distribution policies; local production and innovation systems; and distribution programs through regionalism and multilateralism.
Still, we may only see immediate changes at the national level. Despite work to improve regional regulation (Shadlen & Fonseca, 2021), the overall environment for cooperation has been hampered by a “hollowing out” of inter-state relations and institutions (González González et al., 2021). At the same time, most countries of the region have signed on to the global effort to secure vaccines through COVAX, the Covid-19 Vaccines Global Access, coordinated by Gavi, the Vaccine Alliance, the Coalition for Epidemic Preparedness Innovations (CEPI), and the WHO. The aim has been not only to procure vaccines, but also to facilitate vaccine delivery and the design of cold chains and roll-out plans.
For Latin America, this roll-out at the international level has been slow. Most national governments have struggled to acquire inputs and vaccines from a highly competitive international market. A few individual cases are notable. Chile and Uruguay are regional and global outliers. Chile had achieved at least one dose of vaccination for nearly 90 percent of the population over age 60 and 65 percent of the population over age 45 by mid-May 2021 (Espácio Publico, 2021).
Many questions remain. How did access to medicine deteriorate in Latin America during the pandemic? And were there ways in which innovations could reduce the challenge of sourcing medicine during a global pandemic, be they mimicking the weight of a human hand through an engorged latex glove, launching a new procurement system, or incentivizing local innovators to adapt? How should we think of disruptions in the region from this point forward? What are the models that effectively reduce disruption with a focus on ensuring access, equality, and economic development?
The onset of the pandemic seemed to be a harbinger of shared global destiny. The promise has largely failed to materialize in Latin America. While workers on the front line scramble to get patients critical care, policymakers in ministries and congresses are facing the region’s dependence and debating steps to rebuild local production systems for the future.
Cassandra M. Sweet works on research and policy relating to the political economy of trade. She consults for international agencies, governments, and NGOs and has authored more than a dozen studies on democracy, intellectual property, and innovation.

References
Ali, M.J., Hanif, M., Haider, M.A., Ahmed, M.U., Sundas, F., Hirani, A., Kahn, I.A., Anis, K. & Karim, A.H. (2020). Treatment Options for COVID-19: A Review. Frontiers in medicine, 7, 480. doi:10.3389/fmed.2020.00480
AS/COA. (2021). What portion of each country’s population is vaccinated? Retrieved from https://www.as-coa.org/articles/timeline-tracking-latin-americas-road-vaccination
Benavides-Cordoba, V. (2020). Drug Repositioning for COVID-19. Colombia medica (Cali, Colombia), 51(2), e4279. doi:10.25100/cm.v51i2.4279
Bollyky, T.J., Gostin, L.O., & Hamburg, M.A. (2020). The Equitable Distribution of COVID-19 Therapeutics and Vaccines. JAMA, 323(24), 2462-2463. doi:10.1001/jama.2020.6641
Canedo-Marroquín, G., Saavedra, F., Andrade, C.A., Berrios, R.V., Rodríguez-Guilarte, L., Opazo, M.C., Riedel, C. & Kalergis, A.M. (2020). SARS-CoV-2: Immune Response Elicited by Infection and Development of Vaccines and Treatments. Frontiers in immunology, 11, 569760. doi:10.3389/fimmu.2020.569760
Chazan, G., Solomon, E., Kuchler, H., & Brunsden, J. (2021). Angela Merkel rejects US move to waive patents on vaccines. Financial Times. Retrieved from https://www.ft.com/content/76a05a85-b83c-4e36-b04d-7f44f63e57b0
Correa, C. (2015). The Use of Compulsory Licences in Latin America. In Hilty, R.M, & Liu, K.C. (Eds.), Compulsory Licensing. MPI Studies on Intellectual Property and Competition Law (Vol. 22). Berlin, Heidelberg.
Delgado, D., Wyss Quintana, F., Perez, G., Sosa Liprandi, A.; Ponte-Negretti, C.; Mendoza, I., Baranchuk, A. (2020). Personal Safety during the COVID-19 Pandemic: Realities and Perspectives of Healthcare Workers in Latin America. Int. J. Environ. Res. Public Health, 17(2798).
Espácio Publico. (2021). **bibliography to come**
González González, G., Hirst, M., Luján, C., Romero, C.A., & Tokatlian, G. (2021). Coyuntura crítica, transición de poder y vaciamiento latinoamericano. Nueva Sociedad, 291(Enero-Febrero). Retrieved from https://nuso.org/articulo/coyuntura-critica-transicion-de-poder-y-vaciamiento-latinoamericano/
O Globo. (2021). Anvisa se reúne com entidades em busca de solução para evitar falta de medicamentos nas UTIs. Retrieved from https://g1.globo.com/bemestar/coronavirus/noticia/2021/03/18/anvisa-se-reune-com-entidades-em-busca-de-solucao-para-evitar-falta-de-medicamentos-usados-nas-utis.ghtml
Roffe, P., Tansey, G., & Vivas-Eugui, D. (2005). Negotiating Health: Intellectual Property and Access to Medicines. London: Earthscan.
Sell, S. (2003). Private Power, Public Law: The Globalization of Intellectual Property Rights. Cambridge, UK: Cambridge University Press.
Socal, M.P., Sharfstein, J.M., & Greene, J.A. (2021). The Pandemic and the Supply Chain: Gaps in Pharmaceutical Production and Distribution. American Journal of Public Health, 111(4), 635-639. doi:10.2105/AJPH.2020.306138
Sosa, S. (2021). Tweet. https://twitter.com/drasosa_/status/1390771394938556427. Retrieved from https://twitter.com/drasosa_/status/1390771394938556427?ref_src=twsrc%5Etfw%7Ctwcamp%5Etweetembed%7Ctwterm%5E1390771394938556427%7Ctwgr%5E%7Ctwcon%5Es1_&ref_url=https%3A%2F%2Fcriterio.hn%2Fhospitales-de-honduras-colapsados-sin-medicamentos-para-combatir-el-covid-19%2F
Sweet, C., & Eterovic, D. (2015). Do Intellectual Property Rights Spur Innovation? World Development, 66(February), 665-667.
Tai, K. (2021). Statement from Ambassador Katherine Tai on the Covid-19 Trips Waiver. Office of the United States Trade Representative. Retrieved from https://ustr.gov/about-us/policy-offices/press-office/press-releases/2021/may/statement-ambassador-katherine-tai-covid-19-trips-waiver
WHO. (2018). Brazil highlights treatment for all people with HIV. Retrieved from https://www.who.int/hiv/mediacentre/news/brazil-hiv-treatment-all-plhiv/en/
WTO. (2021). Statement of Director-General Ngozi Okonjo-Iweala on USTR Tai’s statement on the TRIPS waiver. May 6. Retrieved from https://www.wto.org/english/news_e/news21_e/dgno_06may21_e.htm